Theoretical rheo-physics of silk: Intermolecular associations reduce the critical specific work for flow-induced crystallisation
Published in [J. Rheol](https://sor.scitation.org/doi/full/10.1122/8.0000411), 2021
Cite: Charley Schaefer & Tom C. B. McLeish, "Theoretical rheo-physics of silk: Intermolecular associations reduce the critical specific work for flow-induced crystallisation." arXiv.. (2021)
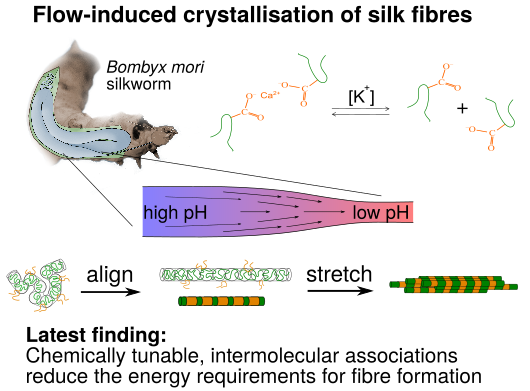
Figure: Our current rheo-physics view on the processing of silk proteins into fibres by the B. mori silkworm
Natural silk is an extraordinary material that remains unmatched by human-made, artificial materials. This is true for both the high-strength and flexibility of the fibres, but also for the processing conditions: Artificial fibres are processed in harsh conditions with high pressures and elevated temperatures, as well as the usage of toxic solvent. Silk, on the other hand, is processed at low pressures and ambient temperatures, and with water as a solvent. An important similarity between the silk protein and artificial materials, is that they are randomly coiled molecules that are deformed by flow, and in response to these deformations self-assemble into a fibre.
To learn the underpinning physical principles used by nature, and eventually apply them to improve man-made materials, I am studying the dynamics of the silk protein in collaboration with Dr. Pete Laity and Dr. Chris Holland at the University of Sheffield and with Prof. Tom McLeish in the Physics of Life group at the University of York. In Macromolecules 2020 we showed that silk can be interpreted as an associating polymer. This class of polymers forms reversible networks and typically has a high viscosity. We discovered, however, that the silkworm manage to weaken the associations by simply increasing the concentration of potassium salt in the solution, which facilitates the processibility of the protein solution. It turned out that the associations not only control the viscosity, but also completely alter the structure of the proteins at a molecular level in conditions of flow. In collaboration with Prof. McLeish, in Phys. Rev. Lett 2021 I showed that these associating chains exhibit conformations ranging from relaxed randomly coiled globules to highly-strained proteins that are stretched in the direction of the flow; the latter are important pre-cursors for fibre crystallisation.
In our latest work, we showed through molecular-dynamics simulations that this phenomenon enables the silkworm to prime a fraction of proteins to crystallise using a very low energy input. Intriguingly, the silkworm further tunes the chemistry of the associations during processing using the acidity. This delicate principle of chemically tunable processing has a high potential for improving the sustainability of industrial flow processing.